The hydrologic cycle is a central phenomenon enabling life on Earth. It is complex on a macroscopic and molecular level and functions interactively with every aspect of our biological, geological, and physical world. Its impact on humanity has anthropological, economic, environmental and social implications that are numerous.

(“Water cycle,” n.d.)
Yet it all starts with one of the simplest of all chemical species – the water molecule. Only 3 atoms in composition, 18 daltons in mass, less than 300 picometers (282 trillionths of a meter) in diameter, its complexity is the subject of numerous books and articles. Under the right conditions, it is a solid, a liquid, a gas, an acid, a base, a neutral atom (although this is rarely true in nature), a ricocheting billiard ball as a gas and/or a component in a complex, flickering lattice of other water molecules in liquid and solid form.
 (Blamire, 2000)
(Blamire, 2000)
Even with this level of complexity, it is impossible to understand the water component of the hydrological cycle without understanding that water loves to mingle with other molecules. If water encounters a solid ionic compound, like the wide range of salts found in soil, streams, rivers, lakes and oceans, it pries the ions apart and engages them in three three-dimensional ballet of solubility. If it encounters an acid, it becomes the hydronium ion in the process of dissolving the acid; if it encounters a base, it becomes the hydroxide ion in the process of dissolving the base. If it encounters reactive gases, like carbon dioxide or sulfur dioxide or nitrogen oxides, it forms carbonic or sulfuric or nitric acids. If it encounters something that is dry, like the surface of a stone or a clump of clay, it erodes and moves some of it to another location, sometimes near its origin and sometimes far away. If it encounters discrete materials, it breaks them down and mingles with them. If it encounters organic compounds, some of which are non-polar and not attracted to the water molecule, it causes them to form droplets or micelles, which are then swept along by the water. With other organic compounds, such as esters and ketones and alkenes, it reacts with them to produce polar products, which can then react with other organic compounds.
In living cells, water is the elixir in which life happens. If a tree, a cell, a human or a cat encounters water, it is sipped up and used to fortify these water-dependent structures, which collapse and turn to dust without its liquid sustenance. Water carries inorganic and organic ions; it carries phospholipids and amino acids; it carries nucleic acids and sugars; it encourages fatty acids to circle the wagons and create cell walls, across which the cell’s supplies are pumped by active and passive portals that open and close for water and its many friends. It encourages DNA to spiral inwards as the nucleotides bond and the sugar/phosphate backbone prickle outwards into the cell’s aqueous soup. Information could not travel if not for the charged molecules that water helps create and carry. But enough about water the molecule. Let’s consider water, the cycle.
Let’s pretend, for an instant, that water “starts” somewhere and continues through the cycle from this starting point. Let’s pretend it starts as precipitation. Forget for a minute that precipitation starts with clouds and clouds start with evaporation and evaporation occurs because of wind, sun, and atmospheric pressure. Forget for a moment that water precipitates as a solid now and then. Let’s just pretend it rains. What happens when it rains? Droplets of water between 0.02 and 0.25 inches in diameter reach terminal velocity of between 5 and 20 miles per hour and strike whatever is beneath them. Each raindrop is rarely pure water; for rain to occur, the vapor in clouds condenses around “a microscopic particle of smoke, dust or salt” (USA Today). In a fascinating calculation, Bob Swanson, a weather editor with USA Today, provides an estimate of the number of droplets that fall in a storm:
“Assuming an average thunderstorm is 15 miles in diameter. Assuming a circular base of the storm, the area of the storm’s cloud base is about 175 square miles. Now let’s assume that .25 inches of rain falls from the storm. This yields a total volume of rainfall of around 175 billion cubic inches. Now if we assume a spherical raindrop, the volume of an average size drop would be about 1/10,000th of a cubic inch. Dividing the total rainfall by the volume of an average raindrop gives a total number of raindrops around 1,620 trillion.”
When one also assumes that each droplet reaches terminal velocity, there is tremendous energy unleashed in a storm. Then think of all the storms that happen and all the energy from all the storms. This is a lot of force dropping out of the sky! When old leaves are struck by raindrops, they are ripped from their homes, becoming compost for new life. If dead things are struck by water, bacteria and molds help decay the creature and turn it back into nutrients, parts of other cycles of nitrogen and carbon and sulfur. If a rock or soil is struck, small amounts are displaced and move away from their source. For evidence of what rain can do, examine the Badlands of South Dakota or the gaping tear known as the Grand Canyon or the alluvial plains of South and North Carolina – created from Appalachian precipitation on mountains that were once five times as high as they are now. Yes, some of this was due to the action of rivers, but the rivers were replenished by rain.
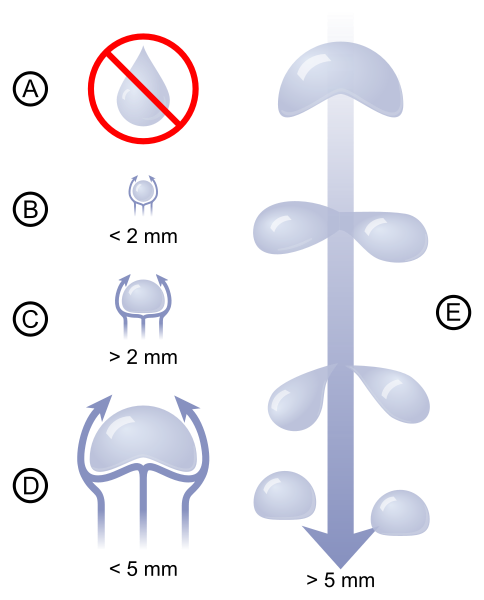
(PBroks13, n.d.)
Different sizes of raindrops:
- A) Raindrops are not tear-shaped, as most people think.
- B) Very small raindrops are almost spherical in shape.
- C) Larger raindrops become flattened at the bottom, like that of a hamburger bun, due to air resistance.
- D) Large raindrops have a large amount of air resistance, which makes them begin to become unstable.
- E) Very large raindrops split into smaller raindrops due to air resistance.
One way that water re-enters the hydrologic cycle is through watersheds, defined as “a land area whose run-off drains into any river, stream, lake or ocean” (USEPA, June 1998, p. 1). Run-off doesn’t only occur on the earth’s surface, though. Of the 332 million cubic miles of water on our planet, 97% of it is salt water and approximately 1.7% of it is groundwater (USGS); only 46% of this is fresh water. This is replenished by seepage into the ground from the various types of precipitation. If we were to dig a perfect hole in the ground, we would find the upper layers a mixture of air and water, but lower layers would become increasingly wet. Eventually, we would reach a level where water occupies all of the space between grains of sand and gravel. This level is called the water table.
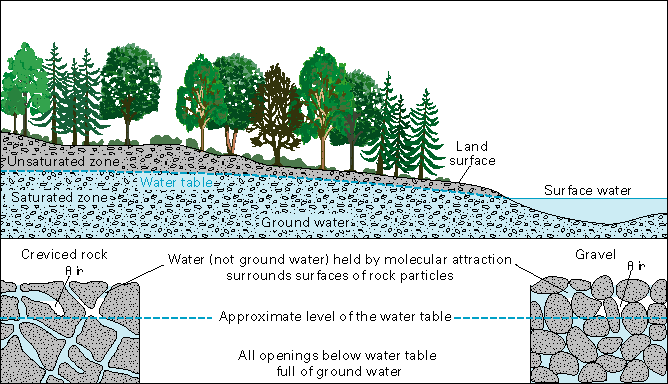 (“Water table,” n.d.)
(“Water table,” n.d.)
Of course, some of the water all courses down streams to rivers and rivers to lakes and lakes to seas and oceans. Some of the water that enters streams and rivers and lakes and oceans weeps out of the ground into these bodies of water, depending on the relative elevation of the water table to the bodies of water in the area. Some of the water in the water table is pumped up for use in homes and factories as well.
The water cycle really gets complex when precipitation falls on and interacts with man-made phenomenon, like roads and highways, or human industries like oil refineries and coal-burning power plants and waste pools for cattle and swine and agricultural fields full of pesticides and herbicides and fertilizers, or human by-products like landfills or untreated waste streams from storm drains. When water, this remarkable molecule, plunges to earth and mobilizes the products of human industry, the entire water cycle becomes contaminated in the process. Water takes our waste and pollutes the rivers, lakes and oceans, creating imbalances in nutrient cycles and killing creatures that depend on a balance between water and salts, nutrients and energy to live their normal lives. Water releases volatile organic compounds from human industry and they become part of our atmosphere. Water mixes with the sulfur and nitrogen oxides and precipitate back to earth as strong acids that change the equilibrium state that nature requires for its magic.
References
Rights for use of the raindrop illustration are granted by Pbroks13 as follows: “I grant anyone the right to use this work for any purpose, without any conditions, unless such conditions are required by law.”
PBroks13. (Artist). (n.d.). Raindrop. [Print Graphic]. Retrieved from http://en.wikipedia.org/wiki/Rain
Bell, J.A. (2005). Chemistry: A project of the American Chemical Society. New York: W.H. Freeman and Co.
Flynn, D.J. (ed). (2009). The Nalco water handbook (3rd ed.). New York: McGraw-Hill Co.
Jacobson, M.C., Charlson, R.J., Rodhe, H., Orians, G.H. (2000). Earth system science. San Diego, CA: Academic Press.
Gruver, J. and Luloff, A.E. (2008). Engaging Pennsylvania teachers in watershed education. Journal of Environmental Education, 40(1), 43–54.
Heimlich, J.E., Oberst, M.C., Spitler, L. (1993). Two H’s and an O: A teaching resource packet on water education. Columbus, OH: ERIC Clearinghouse for Science, Mathematics, and Environmental Education.
Lacosta-Gabari, I., Fernández-Manzanal, R., and Sánchez-González, D. (2009). Designing, testing, and validating an attitudinal survey on an environmental topic. Journal of Chemical Education, 86(9), 1099-1103.
Marques, C., Izquierdo, M., Espinet, M. (2006). Multimodal science teachers’ discourse in modeling the water cycle. Science Education, 90, 202–226.
Sträng, M., and Åberg-Bengtsson, L. (2010). “Where do you think water comes from?” Teacher-pupil dialogues about water as an environmental phenomenon. Scandinavian Journal of Educational Research, 54(10), 313-333.
Walker, M., Kremer, A., Schluter, K. (2007). The dirty water challenge. Science and Children, July, 26-29.
Winter, T.C., Harvey, J.W., Franke, O.L., Alley, W.M. (1998). Ground water and surface water: a single resource. Denver, CO: U.S. Geological Survey.
Retrieved from http://ga.water.usgs.gov/edu/watercyclesummary.html
Retrieved from http://www.srh.noaa.gov/srh/jetstream/atmos/hydro.htm
Retrieved from http://water.chemistry2011.org/web/iyc/experiments
Retrieved from http://www.science.uwaterloo.ca/~cchieh/cact/applychem/waterchem.htm
(n.d.). Water graphic. [Web Graphic]. Retrieved from http://news.cisc.gmu.edu/images/watergraphic.jpg
(n.d.). Water table. [Web Graphic]. Retrieved from http://ga.water.usgs.gov/edu/earthgwaquifer.html
Retrieved from http://ga.water.usgs.gov/edu/watercyclegwstorage.html
Blamire, J. (Artist). (2000). Water molecule. [Web Graphic]. Retrieved from http://www.brooklyn.cuny.edu/bc/ahp/SDgraphics/PSgraphics/SD.PS.LG.Water.html
Retrieved from http://www.usatoday.com/weather/resources/askjack/waskrain.htm
